Keywords: Ecology, Drosera rotundifolia, Russia.
Carnivorous plants found in oligotrophic habitats capture arthropods to obtain nitrogen and other mineral substances (Brewer, 1998; Chandler & Anderson, 1976a; Krafft & Handel, 1991; Masing, 1959; Redbo-Torstensson, 1994). The contents of different mineral substances in the soil can significantly influence the characteristics of plants (Chandler & Anderson, 1976a; De Ridder & Dhondt, 1992; Redbo-Torstensson, 1994). Various authors have established the abundance of carnivorous plants in the moist habitats (Kats, 1941; Dixon & Pate, 1978; Aldenius et al., 1983; Brewer, 1998).
The positive connection between the number of caught insects, the size of the plant, and the possibility of its flowering was shown for Drosera species by Kraft & Handel (1991) and Redbo-Torstensson (1994). Many authors have reported on the dependence of the possibility of flowering, the intensity of vegetative growth, and the success of insect-catching on the environmental factors (Dixon & Pate, 1978; De Ridder & Dhondt, 1992; Redbo-Torstensson, 1994). This is contrary to Brewer's (1998) opinion.
Many publications about Drosera species are connected with the study of the influence of insect-eating on Drosera rotundifolia morphology (Krafft & Handel, 1991; Balandin & Balandina, 1993; Redbo-Torstensson, 1994), and also with the physiological and biochemical aspects of the carnivory for Drosera species in the laboratory (Kellermann & Raumer, 1878; Darwin, 1875; Busgen, 1883; Poretskij, 1914; Chandler & Anderson, 1976a; Chandler & Anderson, 1976b; Pate & Dixon, 1978; Dixon et al., 1980; Thum, 1986). A discussion on some broader aspects of D. rotundifolia ecology is also found in Thum (1986).
However, the experiments in the laboratory described in the majority of cited papers, do not take into account many factors (for example wind, strong rains and overheating) that have an essential influence on the vital functions of carnivorous plants (Chandler, Anderson, 1976a; Hanslin and Karlsson, 1996).
This field-based research project investigates the ecology of Drosera rotundifolia, with an emphasis on studying the influence of the environment on the plant's morphophysiological characteristics.
In July-August of both 2000 and 2001 the flora of fifty-one islands of the Keretskiy archipelago and Keev bay (the White Sea) was investigated during Moscow South-West High School expeditions. Drosera rotundifolia was found at 31.4% of the investigated islands. This implies that D. rotundifolia is a species typical for the study area, but its distribution is probably limited by various environmental factors.
The investigation of the morphophysiological characteristics of D. rotundifolia at
the various ecotopes was carried out at the northern part of the Karelian coast of the
White Sea during July 23-August 11, 2001 (Figure 1).
These sites were laid in random manner on the populations of D. rotundifolia.
Seven of these spots were situated on the islands in Keev bay and in the Keretskiy
archipelago, and the others were situated at the continental lakeside. The surface area of
all the areas were 0.04 sq m, except for one which was 0.02 sq m, and another which was
0.01 sq m. There were 222 D. rotundifolia plants in the sample area. During the
investigation we also recorded all discovered plant species including mosses and lichens.
For each D. rotundifolia plant in our sample, we measured the leaf blade diameter
of the largest leaf, the length of its petiole, the diameter of the leaf rosette, and (if
present) the length of the flower stalk (see Figure 2). We also recorded the number of
captured insects (see Figure 3), and the number of developing, active, and dead leaves for
each plant.
We calculated the fractions of plants with caught insects, and the fractions of plants with developing leaves in the total number of D. rotundifolia plants on each site. We also estimated the capturing surface S of D. rotundifolia as S=3,14*d*d*N/4, where d is the diameter of the largest leaf blade, and N is the number of active leaves per plant (see Figure 4). (The largest leaf blade diameter was used to estimate the size each leaf would become at its point of maximum development.) Indicator-species plants observed at each site were used to estimate the site's environmental factors.
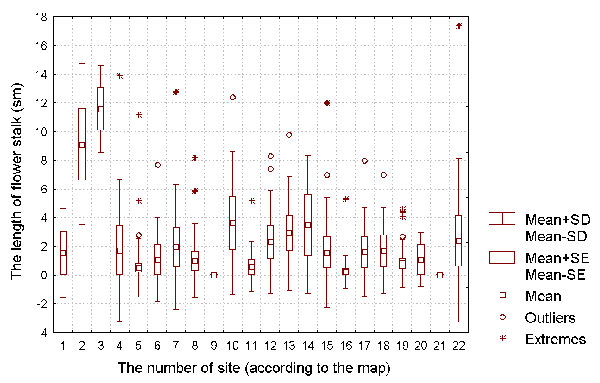
It is well known that a D. rotundifolia seedling can reach its full size within one vegetative season (Thum, 1986; Kraft & Handel, 1991). As such, we can use the size of the vegetative parts of each plant as a characteristics of the intensity of its vegetative growth, and the length of flowering stalk as a characteristic of the plant's reproductive intensity. The number of insects caught by each plant can be used as the characteristic of the success of insect catching by the plant.
A factorial analysis of the site-averaged values of D. rotundifolia morphophysiological characteristics was carried out (see Figure 5). Cluster and discriminate analyses of morphophysiological characteristics for each of observed D. rotundifolia plants were made. We also used the t-test for independent samples to compare average leaf length and average catching surface under different environmental conditions. The distribution of the plants into clusters was tested by performing a Chi-square goodness of fit test to the Poisson distribution. The parametric correlation analysis of our data was carried out. The statistical data processing was performed using the STATISTICA program package (StatSoft, Inc.).
A factorial analysis of the spot-averaged values of D. rotundifolia morphophysiological characteristics was carried out (Figure 2). Two factors completely describing the plant development were found. Factor #1 describes the intensity of reproduction and vegetative growth of D. rotundifolia and the success of it insect catching. Factor #2 describes the spot-averaged quantity of developing leaves (which are not unfolded and so can not catch insects) and the percentage of plants with such type of leaves located on this spot.
The correlation analysis of the obtained data shows that the success of insect catching by D. rotundifolia, intensity of its reproduction, and vegetative growth are related to each other (N=222, r=0.48-0.61, p<0.05). This conclusion confirms the data obtained by Krafft & Handel (1991) and Redbo-Torstensson (1994).
The increase in a density of D. rotundifolia on any spot leads to the decrease of the number of plants with captured insects at the spot (N=22, r =-0.50, p<0.05). This suggests intrapopulation competition for insects. As the density of plants on a spot increases, the length of their active leaves (i.e. that are capable of capturing insects) decreases (N=22, r =-0.50, p<0.05). Thus the overlapping of active leaves is prevented, allowing for decreased competition for insects within a population.
Most of the D. rotundifolia grew on sphagnous spots (36.4% of the 22 investigated populations) or on bogs situated on the heights formed by rocky outcrops (27.3% of the 22 investigated populations). Populations were also found on typical raised bogs (13.6%); in sparse waterlogged stands of conifers with Betula nana (9.1%); on a stony ground (9.1%); and once on a moist track (4.5%). All these ecotopes can be characterized by treeless or sparse pine woods, and little or no slope. In such exposed environments, plants have little wind protection or shading. The exposure to the wind may increase the probability of catching flying insects, while the high light levels promote active leaf formation (Masing, 1959; Chandler & Anderson, 1976a; Redbo-Torstensson, 1994; Brewer, 1998).
Drosera rotundifolia usually grows in association with Vaccinium oxycoccus, Rubus hamaemorus, Empetrum hermaphroditum and Sphagnum fuscum (the various species of Sphagnum occur at 90.9% of the sites).
In the cluster analysis of morphophysiological characteristics, all the investigated plants were divided into seven clusters, one of which included about one third of the total plants. For ten of the investigation spots, more than 50% of the plants were included in this large cluster. In most cases, plants from the a single spot distribute themselves more or less uniformly into 2-4 various clusters. (Chi-square=242.3, 11 degrees of freedom, P=0.0). According to results of discriminant analysis only 39.6% of the total number of the investigated instances of D. rotundifolia are grouped by morphophysiological characteristics according to their location. This confirms there is no significant influence of the environment on the morphophysiological characteristics of D. rotundifolia in our study area.
The largest active D. rotundifolia leaf had a greater diameter if Andromeda polifolia is present, than if A. polifolia is absent (0.4±0.03 cm and 0.3±0.01 cm, respectively). Redbo-Torstensson (1994) noted the increase of the A. polifolia density after the addition of nitrogen in the soil, the occurrence of A. polifolia probably indicates increased soil nitrogen levels. As such, it is likely that the size of D. rotundifolia depends positively on the nitrogen content in the soil.
The leaf-trapping surface area of D. rotundifolia is greater in the presence of Carex pauciflora than when C. pauciflora is absent (1.14±0.16 and 0.62±0.05 respectively). Carex pauciflora is considered to be a plant typical of damp ecotopes (Katz, 1941), so it follows that the total leaf trapping area of D. rotundifolia depends positively on a moistness of its ecotope. Such dependence can be hypothesized as being due to the increased water demand of a larger, tranpirating leaf surface that is covered by secretory glands. This hypothesis explains the observations of many authors (e.g. Katz, 1941; Dixon & Pate, 1978; Aldenius et al., 1983; de Ridder & Dhondt, 1992; Brewer, 1998; and others) about the confinement of carnivore plants to damp ecotopes.
The number density of the plants in the investigated spots varied from 75 m^-2 up to 925 m^-2, and the average value was 256±43.8 m^-2. Redbo-Torstensson (1994) reports densities of 380-520 m^-2. The disparity in number densities can probably be explained by the fewer types of ecotypes Redbo-Torstensson investigated. Thum (1986) recorded 405 plants m^-2 at a small silted bog in Germany -- this suggests that the density of D. rotundifolia varies considerably according to its environment. This fact suggests that the density of D. rotundifolia varies considerably according to its environment.
1. The success of insect catching, intensity of reproduction and vegetative growth of D.
rotundifolia, and the quantity of its developing leaves completely characterize the
development of this species.
2. There is an intrapopulation food competition within populations of D. rotundifolia.
3. Usually D. rotundifolia grows in raised bogs and waterlogged ecotopes.
4. The size of D. rotundifolia depends positively on the nitrogen content in the
soil.
5. The total prey-trapping leaf surface area of D. rotundifolia depends
positively on the moisture level of its environment.
6. The number density of D. rotundifolia varies considerably, depending on its
environment.
Figure 1: The region where D. rotundifolia was investigated. The study spots are indicated with numbers.
Figure 2: The length of flower stalk (with flowers) of D. rotundifolia plants for each of the investigated sites.

Figure 3: The number of caught insects per D. rotundifolia plant for each of the investigated sites.
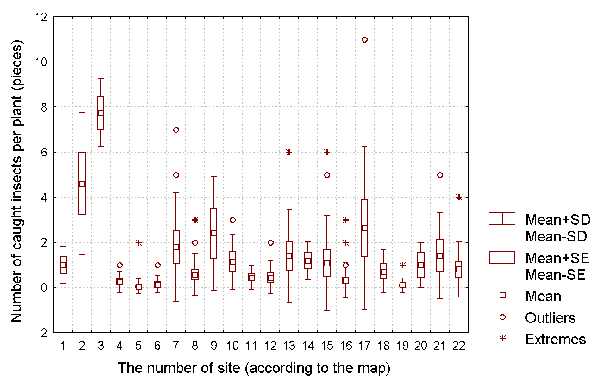
Figure 4: The capturing surface of D. rotundifolia plants for each of the investigated sites.
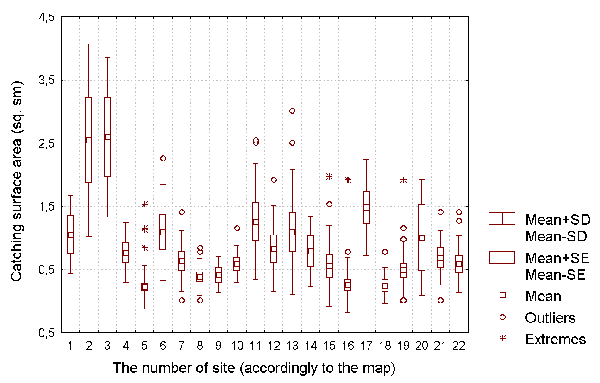
Figure 5: The results of factorial analysis of the spot-averaged values of D. rotundifolia morphophysiological characteristics.
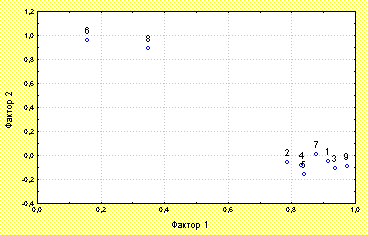
The morphophysiological characteristics of D. rotundifolia are marked with numberes:
1- the diameter of the blade of the greatest active leaf
2- the petals length of the greatest active leaf
3- the diameter of the leaf rosette
4- the number of insects per plant
5- the number of active leaves per plant
6- the number of developing leaves per plant
7- the length of the flower stalk
8- the percentage of plants with developing leaves
9- the whole square of catching surface of the plant
The investigations were carried out in the framework of Moscow South-West High School. I am indebted to my supervisor Dr. Biol. Sc. Shipunov A.B. and all teachers and pupils who helped me in field work.
Aldenius J., Carlsson B., Karlsson S. 1983. Effects of insect trapping on growth and nutrient content of Pinguicula vulgaris L. in relation to the nutrient content of the substrate. -- New Phytologist 93: 53-59.
Balandin S.A., Balandina T.P. 1993. Drosera rotundifolia. -- Biologicheskaja Flora Moskovskoj oblasti 9: 31-38.
Brewer J.S. 1998. Effects of competition and litter on a carnivorous plant, Drosera cappilaris (Droseraceae). -- American Journal of Botany 85 N 11: 1592-1596.
Busgen M. 1883. Die Bedeutung des Insektenfangs fur Drosera rotundifolia L. -- Botanische Zeitung 41 N 35: 569-577.
Chandler G.E., Anderson J.W. 1976. Studies on the nutrion and growth of Drosera species with reference to the carnivorous habit. -- New Phytologist 76: 129-141.
Chandler G.E., Anderson J.W. 1976b. Uptake and metabolism of insect metabolites by leaves and tentacles of Drosera species. -- New Phytologist 77: 625-634.
Darwin C. 1875. Insectivorous Plants. John Murray, London, pp. 145-154.
De Ridder F., Dhondt A.A. 1992. A positive correlation between naturally captured prey, growth and flowering in Drosera intermedia in two contrasting habitats. -- Belg. Journ. Bot. 125 N 1: 33-40.
Dixon K.W., Pate J.S. 1978. Phenology, morphology and reproductive biology of the tuberous sundew, Drosera erithrorhiza Lindl. -- Australian Journal of Botany 26: 441-459.
Dixon K.W., Pate J.S., Bailey W.J. 1980. Nitrogen nutrition of the tuberous sundew, Drosera erithrorhiza with special reference to catch of arthropod fauna by its glandular leaves. -- Australian Journal of Botany 28: 283-297.
Hanslin H.M., Karlsson P.S. 1996. Nitrogen uptake from prey and substrate as affected by prey capture level and plant reproductive status in four carnivorous plant species. -- Oecologia (Berlin) 106: 370-375.
Kats N.J. 1941. Bolota i torfjanniki. Nauka Press, Moscow, pp. 6-10, 53-61.
Kellerman C., Raumer E. 1878. Vegetationsversuche an Drosera rotundifolia mit und ohne Fleischfutterung. -- Botanische Zeitung 36 N 14: 209-218.
Krafft C.C., Handel S.N. 1991. The role of carnivory in the growth and reproduction of Drosera filiformis and D. rotundifolia. -- Bulletin of the Torrey Bot. Club. 118 N 1: 12-19.
Masing V. 1959. Huulheum - Darvin Lemmikmaim. -- Eesti loodus N 6: 354-359.
Pate J.S., Dixon K.W. 1978. Mineral nutrition of Drosera erythrorhiza with special reference to its tuberous habit. -- Australian Journal of Botany 26: 455-464.
Poretskij S.A. 1914. Khichshnije rastenija , Mysl Press, St.-Petersburg, pp. 12-15.
Redbo-Torstensson P. 1994. The demographic consequences of nitrogen fertilization of a population of sundew, Drosera rotundifolia. -- Acta Bot. Neerl. 43 N 2: 175-188.
Thum M. 1986. Segregation of habit and prey in two sympatric carnivorous plant species, Drosera rotundifolia and Drosera intermedia. -- Oecologia 70: 601-605.